Table of Contents
Water Filtration
I lived in NYC, but now reside in Peru, so there are two main sections below.
Water Filtration in NYC
motive
Aside from the usual suspects, flouride and plastics:
The Types of Plastics Families Should Avoid, nytimes.com Callahan 2022
Migration and potential risk of trace phthalates in bottled water: A global situation, Luo et al 2018
Phthalates and diet: a review of the food monitoring and epidemiology data, Serrano et al 2014
Exposure assessment issues in epidemiology studies of phthalates, Johns et al 2015
Reproductive and developmental toxicity of phthalates, Lyche et al 2009
method
To filter tap water, you can buy large tea bags and fill them with a mix of the following:
1) activated carbon
2) “nuclear grade” mixed bed DI resin (aka ion exchange resin)
The activated carbon will help remove many toxins, including chlorine, chloramine, and endocrine disrupters, such as BPA and other bisphenols and pthalates, that leach from plastics. 1 2 3 4
The resin will remove chlorine, chloramine, and any other ionic minerals. Just to be sure, you can replenish the beneficial minerals with a daily multivitamin, or just eating some food can work wonders. The resin will also remove flouride, but weakly due to flouride being a low-selectivity anion.
Dow states: “Fluoride is a very low-selectivity anion so it cannot be removed from water without first removing all of the higher-selectivity anions like chloride or sulfate. Anions are removed according to their selectivity as described in the table of selectivity data. Fluoride can be selectively removed with activated alumina. There are many suppliers available for this media.” However, activated carbon will help protect the ion exchange resin from chlorine: “Used in combination with activated carbon for the added ability to remove of organic chemicals and parasites and to protect the resin from chlorine.” Source Reducing the PH by way of adding ascorbic acid (Vitamin C) to the water helps in removal of chloramines +1 and fluoride, by way of carbon and exchange resin, respectively.
Should I be adding activated alumina? Will activated alumina interact with the carbon or the resin, or add anything back to the water?
Brian from custompure.com answered:
“I’m assuming you are looking for something portable, as you are using now, to remove fluoride. I have good news for you: Mixed bed resin will remove fluoride. Activated alumina, also, will only work within a specific pH range, and is less effective than the mixed bed ion exchange resin. Link: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2006.394/pdf
Out of curiosity, how do you know when to change the ion exchange resin? Are you using a TDS meter?
If you are looking for something to remove contaminants for water in your household, we do offer systems similar to (but smaller than) the ones we have installed in stores like PCC.”
Brian also added:
“I have another note about fluoride regarding selectivity: Stronger bond (high selectivity) ions will kick off weaker bond ions. What this means is that, at first, your resin is a free for all, grabbing everything it can. Then, when all of the binding sites are taken up, the high selectivity stuff will start kicking of the low selectivity stuff. So as you continue to introduce more contaminants (by adding new water when you refill your container), more and more low selectivity ions will be kicked off and replaced by the high ones. This is partially why I asked about a TDS meter- by the time you can taste a difference, you’ve already kicked off virtually all of the fluoride ions (fluoride doesn’t have a flavor or a smell).”
Sodium hydroxide is added to NYC water to increase the PH to 7.3. Sodium hydroxide is also used to flush out and renew ion exchange resin: “For anion resins, regeneration typically involves treatment of the resin with a strongly basic solution, e.g. aqueous sodium hydroxide. During regeneration, the regenerant chemical is passed through the resin, and trapped negative ions are flushed out, renewing the resin exchange capacity” Source. Adding ascorbic acid (the actual Vitamin C) to sodium hydroxide produces sodium ascorbate (C6H8O6 + NaOH → C6H7NaO6 + H20). Sodium ascorbate is the most bio-available form of vitamin C (it is approved for use as a food additive in the EU, USA, Australia and New Zealand), although it could exert pro-oxidative effects on DNA. I assume the amounts involved will be miniscule. PH of 7.3 is hardly a basic solution, so I will skip this step unless the filtering isn't what I expect (after I get a TDS meter).
What happens to chloramines in an ion exchange system?
“Chloramines are formed by the addition of chlorine to ammonia according to the following equilibrium equations:
Cl2 + H2O « HOCl + H+ + Cl-
HOCl + NH3 « NH2Cl + H2O
HOCl + NH2Cl « NHCl2 + H2O
HOCl + NHCl2 « NCl3 + H2O
Since these reactions are in equilibrium, it is possible that free chlorine will be present under certain conditions of pH, temperature and the ratio of chlorine to nitrogen. Free chlorine will degrade an ion exchange resin. This occurs primarily via decrosslinking in a cation resin and defunctionalization in an anion resin. In addition to the free chlorine that is formed when the equilibrium is shifted on these equations, the chloramines, while less oxidative than chlorine, will oxidize the anion resin functional group. Chloramines and free chlorine should be removed prior to ion exchange resin beds. Degradation products of these reactions include ammonia, hydrochloric acid, nitrogen, and in the case of anion resin oxidation - trimethylamine. The ionic species will likely be removed from the water by the downstream ion exchange resin and seldom have an impact on product water quality.” Source
“If water is held in the carbon block for longer period, microorganisms can grow inside which results in fouling and contamination. Silver nanoparticles are excellent anti-bacterial material and they can decompose toxic halo-organic compounds such as pesticides into non-toxic organic products.” Source
The carbon tea bag can be reused up to 50 times, although I'm not sure how many times the ion exchange resin can be reused. I will be trying out a TDS meter. Ok, a long time has passed and I finally got around to getting results from a couple TDS meters:
stanley stainless steel bottle
43 ppm tap
32 ppm after 8 hrs
annie's place with terrible tasting water
34 ppm after 8 hrs
31 ppm after new filter + 5 hrs
39 ppm tap
38 ppm after 2 hrs
36 ppm after 6 hrs
18 ppm after 12 hrs, very low volume remaining in bottle
gaiam stainless steel bottle
32 ppm after 12 hrs
41 ppm tap
glass bottle
29 or 33 ppm after 1 day depending on which tds meter
22 or 25 ppm next day half volume
It seems the tea bags are a somewhat effective, but require a long time to filter the water. I will next try taking a bottle with me, so that it gets juggled around. Maybe the agitation will speed up the filtering process. However, the numbers above, even at 18ppm, is rather poor based on the numbers I remember reading about in a forum, where the forum was about more sophisticated filters including reverse osmosis. My source for carbon is “Marineland black diamond media premium activated carbon”. My source for ion exchange resin is “Puroflo Mixed Bed DI Nuclear Grade” part number WS-MBDI-NG.
After taking a bottle with me cycling, the ppm on both tds meters was 3!!! That means that shaking the water up moved the molecules around sufficiently so that they eventually made contact with the media. Also, the media had already been used in my water bottle for two weeks (although I had a larger glass bottle with a teabag filter, that I used as a first stage, pouring water from it to my drinking bottle). The results are therefore comparable with a reverse osmosis filter.
My original thought was that water molecules in an unshaken container were always moving around much like in a gas, but at much closer range. This does not appear to be so much the case. What gave me this idea, is that food coloring dropped into water disperses rather quickly. However, this is a different phenomena. I believe dispersion happens because of the Van-der-waals forces between the food coloring and the water. The higher energy state and the ensuing need for entropy disperses the food coloring evenly among the water molecules. Therefore, it appears that in order to speed up the process of getting the non-water molecules over to the tea bag (to get attached to the media), some shaking is required.
My solution was to get a small 3 watt submersible fish-tank pump for $3 on ebay, which I put into a gallon glass jar (the kind used for pickles) along with the tea bag. I measure the PPM after the water filters overnight. Now I can tell when the filter material is used up, because the lowest PPM attainable quickly increases. The lowest attainable has shown anywhere from 0 to 2 ppm. When the tea bag gives up the ghost, the lowest quickly increases to 7-15ppm. I think this happens after about 10 gallons / 40 liters, but I haven't been keeping track.
In hoping to have the filtration take less time, and possibly use the media more uniformly, I've wanted to try putting the pump in line with a refillable filter cartridge, such that the water is forced through the media. However, I can't seem to find one that is less than the standard 10 inch length, and that will not fit well in a glass jar. It would have to be a very big glass jar and I'm not sure where to find such a beast.
On ebay I saw some very small funnels for a dollar, that I hope will fit on the intake of the water pump. The idea is that the tea bag will get stuck in the funnel and the water will be forced through it. So far, I've been having the tea bag circle along in the current created by the pump, by placing the pump at a higher level than the tea bag. The tea bag floats around close to the bottom. Without the funnel, if the tea bag gets caught on the intake of the pump, the small surface area of the intake, covered by the tea bag paper, does not allow a sufficient amount of flow.
What Does a TDS Meter Not Measure? source
“ Because TDS is an aggregate measure of charged compounds in water, uncharged things like motor oil, gasoline, many pharmaceuticals, and pesticides do not contribute to a TDS measurement. For example, the glass on the left in this article's header image contains deionized water with Malathion (an organophosphate pesticide) dissolved into it at 100 times higher concentration than allowed by the EPA for drinking water, and the TDS probe reads 000. ”
I thought the whole point of anything being able to dissolve in water, was that it needed to be ionic: that's why oil and water don't mix? Well, I'm wrong. From wikipedia:
“ Dissolved organic solids, such as sugar, and microscopic solid particles, such as colloids, do not significantly affect the conductivity of a solution, and are not taken into account. ”
One of the main reasons I'm using activated carbon is to help remove plastics. Yet when I put a tea bag of carbon in my camelback, the water still tastes like plastic. I need to try and see if the tube or bladder causes the most effects. The carbon does keep the plastic taste from forming in my cycling water bottle. They have inline carbon filters for the hose line, and users leave positive comments.
Update: I spent a week in Somersworth, NH, and the tap water quality is horrible, in terms of a TDS reading of 150ppm, compared with nyc 40ppm. A single tea bag filter (working with the pump) was only good for purifying 6 liters of water to 1ppm. After that, the next 6 liters went no lower than 82ppm. I continued filtration with an unused filter.
Update: If you want to remove flouride, most TDS meters will not be that helpful, because flouride is added to water at 1ppm. Most TDS meters do not have enough resolution to estimate the percentage of flouride that is removed by your filter. You need a TDS meter with a resolution of at least 0.1 ppm.
consumer products
zero water filter
ZeroWater uses a five-stage Ion Exchange filtration to remove 99.6% of detectable dissolved solids. They claim that their filters produce water that is a similar purity level to the water from a reverse osmosis system.16 They remove antimony, arsenic, barium, beryllium, cadmium, chromium-3, chromium-6 (hexavalent chromium), copper, iron, lead, manganese, mercury, selenium, silver, thallium, zinc, asbestos, chlorine, cyanide, fluoride, nitrate, nitrite, and PFAS.17 The main page of this company’s website claims to be the only filter NSF Certified filter to reduce PFAS. This seems like a good option. becauseturtleseatplasticbags.com
The problem with the Zero unit, you see, is that pure water seeks to become contaminated. It will hungrily absorb CO2 from the atmosphere, forming carbonic acid, as well as any contaminants in the air. More dangerous is the fact that the Zero Water Filter removes 100% of the chlorine form the water. If your municipality did that, your community would suffer another yellow fever, legionella or other bacterial/viral disease outbreak.
Bacteria will grow in water that has no chlorine in it. If you use the Zero Water Filter remember that the filtered water is chlorine free. Also remember, ion-exchange resin is also a great medium for growing bacteria. The TDS meter on this device measures electrical resistivity or conductivity. It has no capacity to measure algae, fungi and microorganisms in your water.
The purification technology in this unit itself is sound, but the product has no technology to keep the water pure once it is made so. In water treatment science it is more expensive to keep water pure than it is to make it pure. I am willing to bet that if you do not sterilize your pitcher daily, you have bacteria growing in the system. wateristhenewgold.com
While it may sound impressive that 91% of fluoride continues to be filtered out after 40 gallons, they tested flouride by itself. Results would be much poorer if fluoride was mixed with other contaminants with higher selectivity to their ion exchange resin, as fluoride would be knocked off the adsorption site.
microorganism load
In order to reduce the microorganisms growing inside a filter cartridge, it's best to have a large family that will use up its filtering capacity quickly (so that the water filter gets replaced frequently).
Comparing the “zero water filter” with the “ion-exchange + carbon tea bag” (see section “method” above):
Since you will more often discard a tea bag than a larger filter cartridge, maintaining sanitation of the filter media is easier with tea bags. A glass jar is also easier to maintain sanitary, compared to a plastic carafe. The only weakness in filtering with a tea bag is the need for a small water pump.
Keep the pitcher with its filter cartridge in the fridge also reduces microorganism growth. Mold's ability to grow reduces with temperature and humidity, and the environment in the fridge is designed to be low humidity.
maintaining water purity after filtration
Pure water will strongly attract any impurity, including contaminants in the air. In order to keep purified water pure, keep in a glass container with a sealed lid. Another option is to dirty the water so it is no longer pure! Add some sea salt, lemon juice, tea, cinnamon sticks, cloves, etc.
boiling pure water
It's not advisable to boil pure water, because it will become contaminated quickly. If you must, you can use a stainless steel tea kettle to boil the water, as stainless steel is highly unreactive.
demineralization controversy
Demineralized water has been claimed to have negative health effects because of a reduction in beneficial minerals.
http://jwh.iwaponline.com/content/6/4/433
A systematic review of analytical observational studies investigating the association between cardiovascular disease and drinking water hardness (2008)
“This study found significant evidence of an inverse association between magnesium levels in drinking water and cardiovascular mortality following a meta-analysis of case control studies. Evidence for calcium remains unclear.”
http://journals.sagepub.com/doi/abs/10.1097/01.hjr.0000214608.99113.5c
Review of epidemiological studies on drinking water hardness and cardiovascular diseases (2006)
“Many but not all ecological studies found an inverse (i.e., protective) association between cardiovascular disease mortality and water hardness, calcium, or magnesium levels; but results are not consistent.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2854772/
Relationship between Tap Water Hardness, Magnesium, and Calcium Concentration and Mortality due to Ischemic Heart Disease or Stroke in the Netherlands (2009)
“We found no evidence for an overall significant association between tap water hardness, magnesium or calcium concentrations, and IHD mortality or stroke mortality. More research is needed to investigate the effect of tap water magnesium on IHD mortality or stroke mortality in subjects with low dietary magnesium intake.”
http://journals.lww.com/epidem/Abstract/2000/07000/Magnesium_in_Drinking_Water_in_Relation_to.9.aspx
Magnesium in Drinking Water in Relation to Morbidity and Mortality from Acute Myocardial Infarction (2000)
“The odds ratio for death from acute myocardial infarction in relation to water magnesium was 0.64 (95% confidence interval = 0.42–0.97) for the highest quartile relative to the three lower ones.”
https://examine.com/supplements/magnesium “Magnesium deficiencies are common in the western diet because grains are poor sources of magnesium.” People must not be eating much of the following: https://www.dietitians.ca/Your-Health/Nutrition-A-Z/Minerals/Food-Sources-of-Magnesium.aspx
“Epidemiological research in the US, Europe, and Russia suggests health benefits may be associated with at least 20-30 mg/l calcium and 10 mg/l magnesium in drinking water.” Which on average agrees with what is supplied municipally. Source
“Recommendations for magnesium have been put at a minimum of 10 mg/L with 20–30 mg/L optimum; for calcium a 20 mg/L minimum and a 40–80 mg/L optimum, and a total water hardness (adding magnesium and calcium) of 2–4 mmol/L” Source: František Kožíšek, M.D., Ph.D via Wikipedia. Following this recommendation, even if the other elements are not counted, the sum of 25 mg/L of magnesium plus 60mg/L of calcium would have us drinking water that is 2.5 mmol/L. Using 30mg and 80mg, respectively, gives 3.2 mmol/L. Hardness is usually measured by CaCO3 (calcium carbonate). Source 2 mmol/L = 200.17 mg/L of calcium carbonate, which, according to the following table, is very hard water.
Concentration as CaCO3 Indication
0 to 60 mg/L Soft water
60 to 120 mg/L Moderately hard water
120 to 180 mg/L Hard water
180 mg/L and above Very hard water
Following the recommendation would lead to very very hard water, considering that elements besides calcium and magnesium are not included. It's probably safer to just have what's natural. So what is natural? “The hardness of local water supplies depends on the source of water. Water in streams flowing over volcanic (igneous) rocks will be soft, while water from boreholes drilled into porous rock is normally very hard.” Source Also, the mineral content varies greatly depending on the source, where, for example, even if the water is hard, magnesium may not be a part of the water supply (unless added by municipalities).
The recommended daily intake for magnesium is 400mg. A liter of drinking water in the U.S. has about 10mg/L of magnesium. It's recommended to drink 2 liters a day. That's 20mg of Magnesium from drinking water. Um… that's significant? There was a study that showed people drinking demineralized water urinated 20% more. Maybe they liked the water better? I guess I'm going to keep taking my multivitamin and magnesium supplement, and I hope I'll be ok. That along with my raw milk 
fluoride controversy
Developmental Fluoride Neurotoxicity: A Systematic Review and Meta-Analysis (2012)
Although reports from the World Health Organization and national agencies have generally focused on beneficial effects of fluoride (Centers for Disease Control and Prevention 1999; Petersen and Lennon 2004), the NRC report examined the potential adverse effects of fluoride at 2–4 mg/L in drinking water and not the benefits or potential risks that may occur when fluoride is added to public water supplies at lower concentrations (0.7–1.2 mg/L) (NRC 2006).
I'm not willing to stake that fluoride is good at 1 mg/L, and bad at 2mg/L. The ion itself is not used in any natural process by the human body. It is not a nutrient. There is no US RDA. It simply acts as a toxin, at any concentration, and the effects are noticeable when there is enough of it. In the following paper, when it says “no evidence exists”, it means that there hasn't been any scientific research done, not that it has been proven to not be harmful.
Review of Scientific Papers Relating to Water Fluoridation
Beneficial effects
a) Dental caries
• Water fluoridation reduces the prevalence of caries in both children and adults in populations receiving water containing fluoride. Estimates of the mean difference in the proportion (%) of caries-free children place the figure between 14–15% [11, 13]. An estimate of the prevented fraction in adults was 27% [6]. 6, 7, 9, 11, 13, 17, 21, 22
• Water fluoridation has a beneficial effect (on caries) over and above the effect of fluoride from sources other than water. 11
• While water fluoridation reduces caries, topical application of fluoride is a more efficient way of achieving protection (i.e., post-eruptive action – although the relative contributions of pre- and post-eruption are argued). 15, 21, 22
• Differences in caries rates between fluoridated and non-fluoridated communities are smaller than has been found previously. The increased use of other sources of fluoride, such as fluoridated toothpaste, appears to be the reason. 15, 18
• Communities receiving fluoridated water have consistently lower rates of caries than those receiving non-fluoridated water. 6, 15
• Cessation of fluoridation can result in an increase in caries. 9, 12
• There is limited knowledge of the efficacy of fluoride in reducing caries if the fluoride concentration in water is reduced below levels presently considered as optimum. 15
b) Redressing social inequalities
• The evidence for water fluoridation reducing differences, due to socioeconomic status, in oral health is inconclusive. 3, 11, 17, 21, 22
Adverse effects
a) Dental fluorosis
• Dental fluorosis is a demonstrated and acknowledged adverse effect that can arise from fluoridation.9, 11, 13, 22, 24, NFIS 35
• There is a dose-response relationship between fluoride concentration and the prevalence of fluorosis, but the fluoride concentration threshold at which fluorosis becomes evident has not been determined. The prevalence of fluorosis at a drinking-water fluoride concentration of 1 mg/L was estimated to be 48% [11]. 11, 22
• Fluorosis arising in areas with fluoride concentrations close to optimal (ca. 1 mg/L) is usually described as very mild or mild. 13, 24
• In some communities (reported in the European Union and United States) an increase in very mild and mild fluorosis has been reported in communities receiving fluoridated water. 24
• The most likely contributor to the increased prevalence of fluorosis, where reported, is increased use of fluoridated products (particularly adult-strength toothpaste) by children under six years of age. 2, 15
• Sources of fluoride other than fluoridated water, such as fluoridated toothpaste and fluoride tablets, are risk factors for the development of fluorosis. 24
• The association between fluorosis and use of infant formula increases with the level of fluoride in the water supply, but the evidence for this being due to fluoride in the formula is weak. 8
• The total fluoride intake, not just intake from water, of an individual is the true determinant of the risk of fluorosis, and it is important that contributions from all sources are determined. 7, 10
• The most critical period for exposure to fluoride with respect to the development of fluorosis of the upper anterior incisors (those of greatest cosmetic importance) is the period between the ages of 15 and 30 months. 2, 10
• Recent overseas studies have consistently shown that very mild fluorosis does not have an adverse effect on oral health-related quality of life, but severe fluorosis is consistently reported to do so. 4
b) Other adverse effects
• There is no evidence showing that water fluoridation increases the likelihood of hip fractures in the population receiving the water. 2, 7, 11
• Water fluoridation appears to have no effect on the likelihood of bone fracture, reduced BMD, or reduced bone strength, and some evidence suggests tha fluoride concentrations around 1 mg/L may be beneficial. (NB. Reviews 7, 9 and 11 concluded that there was insufficient evidence to assess the effects of fluoridation on bone disorders other than hip-fractures). 5, 13
• There is either insufficient evidence to assess the effect of fluoridation on other adverse effects, or the available evidence indicates there is no influence. 7, 11, 13, 14, 22, 25 NFIS 36
c) Effects from fluoridation chemicals
• There is no evidence that, following the dissolution of fluoridation chemicals during the fluoridation process, any adverse effects will arise from the undissociated chemicals themselves, fluorosilicate species, impurities in the chemicals, or the dissolution of metals in water systems because of a substantial depression in the pH of the water.
http://www.rph.org.nz/content/be2be2c4-4fef-49cf-93d6-172f2c8e4a88.cmr
water pH
Should drinking water be at a certain pH? Well, I'm taking the pH out and making it completely neutral with the ion exchange resins. There is some marketing fad to sell alkaline water. If it's supposed to be basic, then I've got that covered because I brush my teeth with baking soda. The food you eat will have so much more ions in it than the trace amounts in water. Does it matter that it goes in with the wrong pH, if it quickly gets mixed up with the food in your belly?
Related article: Alkaline Water: Benefits and Risks, healthline.com 20190530
Water Filtration in Peru
Whole House Water Filtration
Settling down now, so for me it's time to ammend this old nomadic article with whole house water filtration.
I get municipal water that's not treated with chlorine or flouride. Hurray! I also get water that looks muddy.
Since I'm not needing to clear out chlorine and flouride, I may not have to work as hard?
Challenges
Bacteria
My objective may be to filter out bacteria and anything that would feed bacteria downstream of the filter.
However, even a 0.1 micron filter lets through some smaller bacteria. Please note in the following studies that “filterable bacteria” are bacteria that make it through a fiter:
Quantification of the Filterability of Freshwater Bacteria through 0.45, 0.22, and 0.1 μm Pore Size Filters and Shape-Dependent Enrichment of Filterable Bacterial Communities, Wang et al 2007
Broad diversity of viable bacteria in 'sterile' (0.2 microm) filtered water, Martin W Hahn 2004
Passage and community changes of filterable bacteria during microfiltration of a surface water supply, Liu et al 2019
Legionellae require 0.2 micron filtration. Comparison of membrane filters for recovery of legionellae from water samples, Smith et al 1993
“Sawyer water filters are either 0.1 microns or a 0.02 micron. These are “absolute” filters and NOT nominal. Absolute means there is no pore larger than stated. Nominal means average pore size.” sectionhiker.com
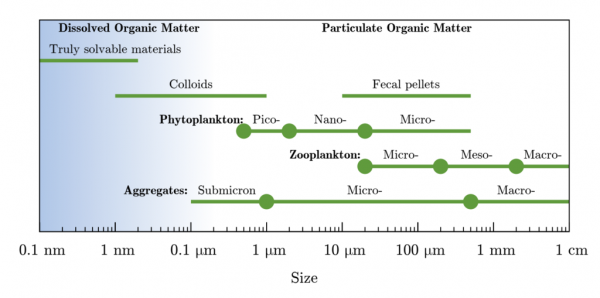
Food for bacteria includes dissolved organic matter (DOM). The size is defined as less than 0.22 microns.
It is generally recognized that the UF process can remove 80–90% of turbidity and 30–40% of dissolved organic matter (DOM) in surface water, and Bu and colleagues used coagulation–ultrafiltration to treat micro-polluted surface water, and the removal efficiency of DOM was 53.8% (Bu et al. 2019). Reverse osmosis (RO) filtration is a common method for the treatment of urban wastewater resources. It has a good effect in treating ions and DOM in wastewater; Lee and co-workers studied the use of RO membranes for wastewater regeneration, and the results showed that RO membranes can remove more than 98% of salt in wastewater, and the removal effect of DOM is more than 80%. Removal characteristics of dissolved organic matter and membrane fouling in ultrafiltration and reverse osmosis membrane combined processes treating the secondary effluent of wastewater treatment plant, Liu et al 2021
Sterilize all the house plumbing?
I'd have to purify all incoming water to starve out bacteria in the plumbing. However, even RO systems leave some 20% DOM dissolved organic matter of small molecular weight.
I could choose to accept any existing colonies of bacteria I have in the plumbing, or temporarily chlorinate the hell out of them to reduce their population, and avoid the vast majority of new bacteria with absolute 0.1 micron filtration.
Ozone filtration makes ozonated water which will carry into the house plumbing. Ozonation is used in many cities instead of chlorine.
Less resources would be used if I can get away with removing TSS (total suspended solids) for whole house plumbing, instead of TDS (total dissolved solids). Leave full water purification for drinking and cooking. An inline shower filter can help reduce dermal absorption of nanoplastics and other toxins.
I could try out a 20“ x 4.5” 0.1 micron whole house filter, if I could just find one for sale. I don't need a high flow rate going into the house because there is an 1100 liter water storage tank.
Viruses
For drinking water, do I need to clear viruses as well?
It is well known that bacteria are major causes of diarrhea transmitted through unsafe drinking water. What is less appreciated are viruses in these same drinking water sources and their impact on human health. Water-transmitted viral pathogens that are classified as having a moderate to high health significance by the World Health Organization (WHO) include adenovirus, astrovirus, hepatitis A and E viruses, rotavirus, norovirus and other caliciviruses, and enteroviruses, including coxsackieviruses and polioviruses. Waterborne Viruses: A Barrier to Safe Drinking Water, Gall et al 2015
We most frequently identified Norovirus NV (14%), Enterotoxigenic Escherichia coli ETEC bacteria (11%), and Campylobacter bacteria (9%) in participants with TD, which differs from the results of a recent review identifying ETEC (33%), NV (15%), and EAEC (13%) as the top three Travelers Diarrhea TD pathogens in Latin America.17
…
Globally, NV is the most common cause of all varieties of diarrhea, accounting for double the number of cases as ETEC, and diarrheagenic E. coli and NV are commonly identified among Latin American travelers with TD. Case–Case Analysis Using 7 Years of Travelers' Diarrhea Surveillance Data: Preventive and Travel Medicine Applications in Cusco, Peru, Jennings et al 2017
While different studies have different results as to the most common cause of diarrhea, I tentatively resolve that viruses are an issue that must be addressed.
Turbidity
Turbidity is caused by both TSS and TDS. Total dissolved solids (TDS) is a measure of the dissolved combined content of all inorganic and organic substances (including tannins) present in a liquid in molecular, ionized, or micro-granular (colloidal sol) suspended form.
Total Suspended Solids (TSS) are particles that will not pass through a 1-1.2 micron filter. Smaller particles that pass through a 1-1.2 micron filter are known as Total Dissolved Solids (TDS).
…
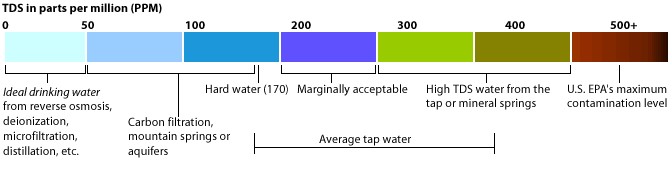
Microorganisms can attach themselves to suspended particles in the water, which can prevent the water from proper disinfection resulting in human health problems. TDS in water does not directly affect human health though high levels could indicate the presence of toxic materials, in Canada the recommendation for drinking water is 500 mg/L TDS mainly for taste and aesthetics.
hmgawater.ca
Despite the above definition for TSS and TDS, I think clay that shows up collecting in your toilet can be below 0.2 microns: “Coarse, medium, and fine clays have size ranges about 2–0.5, 0.5–0.2, and below 0.2 μm, respectively.”. Excerpt from Encyclopedia of Earth Science
Figure 2: TDS levels in ppm (which is equivalent to mg/L), US level of 500 mg/L is the same as Canada
Source: http://www.tdsmeter.com/what-is
TDS Corrosion
Total Dissolved Solids Increase Water Heater Corrosion Many water heater manufacturers place a limit of 500ppm TDS in their warranty.
Salts and metals impart a slight conductivity to water. Through an electrical process called electrolysis, this conductivity will eventually cause metal to rust or corrode. When water is heated, this electrical process can be accelerated.
Most water heaters are made of a steel tank with a porcelain enamel (glass) lining.
It is not always possible to completely cover the inside of the tank, and it’s important to provide metal that can be consumed by the electrical process.
This is where the sacrificial anode rod comes in. By acting as a lightning rod for the corrosion process, the anode rod draws the harmful electrolytic process away from the water heater tank and focuses the corrosion on the anode rod.
Hard Water or soft water that is high in TDS need this sacrificial anode rod to ensure that the electrolysis doesn’t artificially shorten the life of the water heater. premierwatermn.com
After I filter out sediment, then I can get a TDS meter reading and plan accordingly.
You don't have to have an electric water heater tank. You can use a solar water heater and have cheap backup electric showerheads. In my case, the distance of 12 guage cable needed to operate the showerhead is expensive, as I'm far from the power box.
Nanoplastics, Plasticizers and Bisphenols
Nanoplastics, plasticizers such as phthalates, and bisphenols
Even the most remote locations on Earth have rainfall that contains dangerous human-made plastic chemicals. Bad News for Earth: Rainwater Is No Longer Safe to Drink, popularmechanics.com 2022
Available information suggests that inhalation of indoor air and ingestion of drinking water bottled in plastic are the major sources of microplastic exposure.
…
Exposure of human cell lines to MP additives such as phthalates, bisphenols, and organotins causes adverse effects through the activation of nuclear receptors, peroxisome proliferator-activated receptors (PPARs) α, β, and γ, and retinoid X receptor (RXR), leading to oxidative stress, cytotoxicity, immunotoxicity, thyroid hormone disruption, and altered adipogenesis and energy production.
A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens, Kannan and Vimalkumar 2021
Exposure to bisphenols and phthalates, including a BPA replacement, is associated with increased oxidant stress, insulin resistance, albuminuria, as well as disturbances in vascular function in healthy children. Exposure to Bisphenols and Phthalates and Association with Oxidant Stress, Insulin Resistance, and Endothelial Dysfunction in Children, Kataria et al 2017
Murray and Örmeci (2020) investigated the efficiency of filtration to remove nanoplastics <400 nm using different filter pore sizes and materials from 0.02 to 3 μm. Their results showed that 92% ± 3 of nanoplastics were removed from tap water with 0.02 μm filter pore. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process, Arenas et al 2021
I can't find any research for dermal absorption of nanoplastics while showering or bathing. The most recent reviews don't include it. For plasticizers such as phthalates, absorption through air is an important factor, yet they use a shower to end continued absorption for their study.
Updates:
Dermal absorption occurs at a significant rate for phthalates Dermal toxicity elicited by phthalates: Evaluation of skin absorption, immunohistology, and functional proteomics, Pan et al 2014
While studies have shown that direct human exposure to microplastics poses risks to human health, more research is needed to understand the extent of human exposure to microplastics via different pathways. The EU-funded DermPlast project will study the dermal absorption of toxic plastic additives (plasticisers and flame retardants). Assessment of human Dermal exposure to microPlastics additive chemicals and the risk arising from such exposure using innovative 3D-human skin equivalents, ends Nov 2023
Per- and polyfluoroalkyl substances (PFAS)
PFAS Drinking Water Treatment.pdf (2020)
PFAS is best removed by reverse osmosis and multi-stage filtering (sediment + carbon)Assessing the Effectiveness of Point-of-Use Residential Drinking Water Filters for Perfluoroalkyl Substances (PFASs), Herkert et al 2020
PVC Plumbing
There has to be point-of-use filtration because synthetic plumbing leaches all sorts of chemicals into the water supply.
In the present study, the migration of volatile organic compounds (VOCs) from water pipes manufactured of polyvinyl chloride (PVC) has been investigated … in residential areas. … Twenty water samples were collected from houses within Medina Al-Munawarah residential area and were analyzed … The presence of carbon tetrachloride (CTC), toluene, chloroform, styrene, o-xylene, bromoform (BF), dibromomethane (DBM), cis-1,3-dichloropropane (Cis-1,3-DCP), and trans-1,3-dichloropropane (Trans-1,3-DCP) was initially confirmed. The most frequent contaminants found were DBM, CTC, and toluene that were monitored in 55%, 50%, and 45% of samples, respectively. The levels of CTC, Cis-1,3-DCP, and Trans-1,3-DCP were found to exceed the World Health Organization (WHO) limits in 50%, 20%, and 20% of samples, respectively. Leaching of Organic Toxic Compounds from PVC Water Pipes in Medina Al-Munawarah, Kingdom of Saudi Arabia, Shaikh, Hanafiah, and Basheer 2019
Plastic Filter Housing
You would think all filter housing would be made from stainless steel or glass, but most are made of plastic.
Fridge storage of the pitcher reduces plasticizers and bisphenols leaching from the plastic. At least that was my initial assumption. So I started looking up some research on the matter, and found the following studies that are all over the place.
I will be transferring the water to a glass jar after filtration.
Study 1: “To eliminate the threat of leaching toxic bisphenol, water must be kept at or below 25°C.” Adam Moore, PhD interview about his study.
Study 2: “the decrease in concentration of BPA found in plastics stored above 30C over 60 days may be due to increased degradation of BPA in the water samples” Leaching of Bisphonal A (BPA) from Plastic Materials Increases with Storage Time While It's Degradation Increases with Temperature, Uadia and Tunmise 2019
Study 3: The likelihood of contamination of a product with bisphenol A increases at elevated temperatures, e.g., when the product is heated, e.g., by pouring hot milk into a bottle or exposing it to direct sunlight. Moreover, the pH (acidic juices or alkaline liquids) is another factor that contributes to BPA migration.
…
In the case of scientific reports concerning the BPA content in bottled water, it is possible to find results regarding the concentration of this compound depending on the temperature at which the bottled water is stored. However, these data do not give an unambiguous conclusion. There are studies showing an increase in the content of bisphenol A with an increase in storage temperature [32] and contradicting reports that indicate a lack of such correlations [33].
The research regarding BPA migration carried out to date has not allowed us to conclude whether a higher temperature results in increased penetration of bisphenol A into the product. It is similar in the case of the presented research because the concentration of bisphenol A was not always the highest at the highest temperature.
Hard Water Scale Deposits
I noticed the electric tea kettle quickly got a scale deposit, so some anti-scale filtration would be nice.
Air Bubbles
Air coming in with the municipal tap water can cause air hammering which can damage the plumbing.
Filter Types
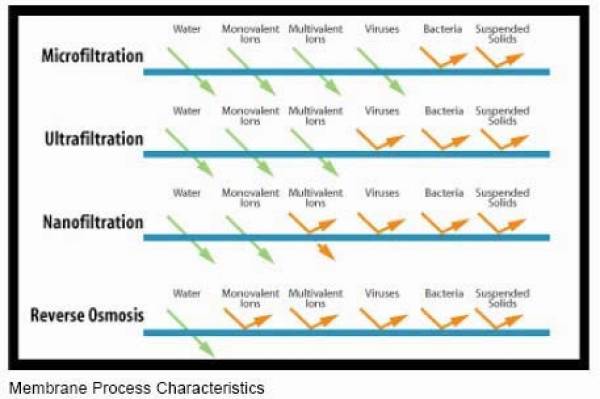
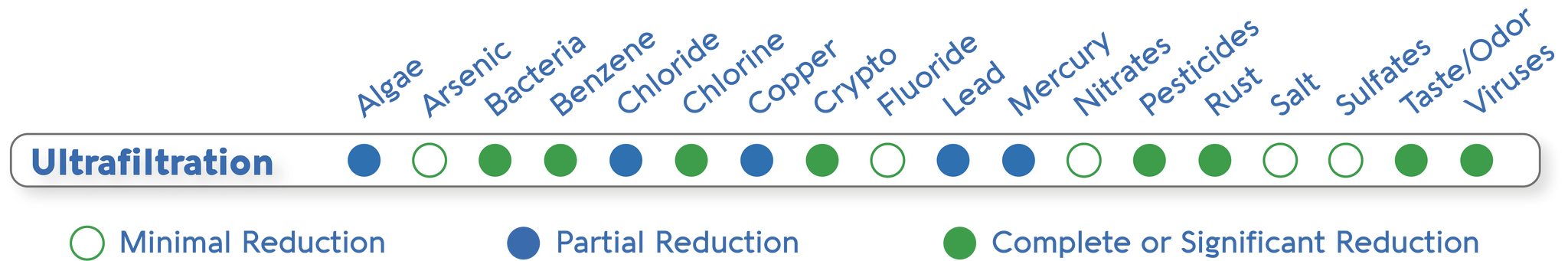
The first four columns of the image below are ion exchange resins (EIR) and activated carbon (C). EIR+C is almost as capable as reverse osmosis and carbon. Both cannot remove benzene, and IER+C removes an insufficient percentage of fluoride.
IER+C is used in the stages of systems such as consumer water filtration pitchers. Some pitcher filters add further stages capable of removing bacteria and viruses as well.
For pitcher filters, the percentage removal of contaminants is advertised for fresh media. Unlike reverse osmosis, percentage removal drops quickly for some contaminants with the age of the carbon media. I suspect the same with the ion exchange resins, since both IER and C function by adsorption.

becauseturtleseatplasticbags.com
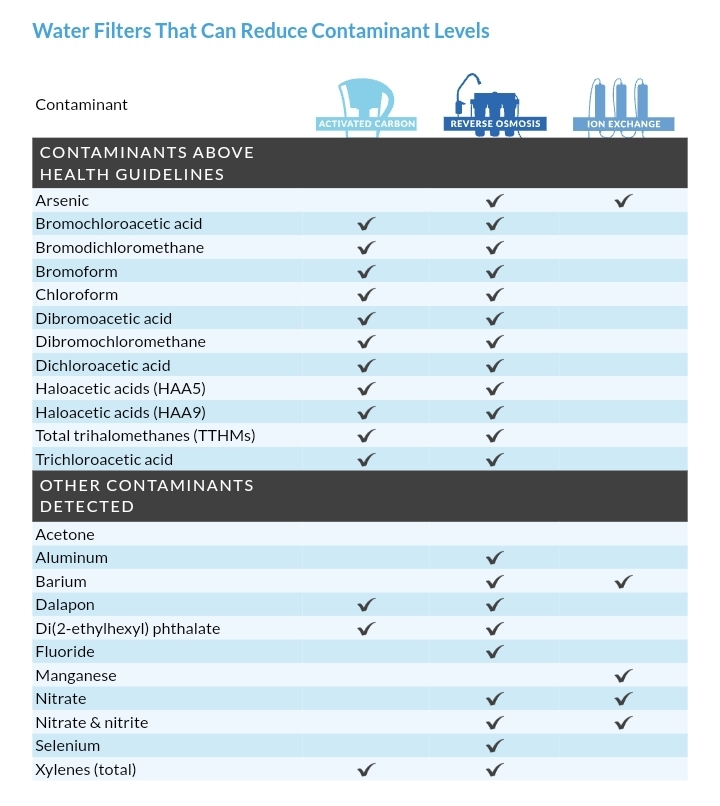
Environmental Working Group 2023
Unfortunately, I can't find a page on the EWG website listing a table of all contaminants and what they are filtered by. EWG has yet to respond to my request. Also asked for any existing database on Quora.
Contaminant Removal by Consumer Brands
Automatic Air Vent
You place an automatic air vent (AAV) at a high point in the plumbing. Air bubbles rise and will exit through the vent. An AAV has a float valve that rises with water to seal the opening.
If a high point is not a convenient place to install the AAV, you can turn a spin down filter upside down, unscrew the ball valve, and screw in the AAV. You may need an adapter if the threads don't match up.
Sediment Spin Down Filter
Indespensible if drawing water from a well or stream where the water is murky. Sediment spin down filters remove all particles greater than 40 microns. The filter is a metal screen lasts a very long time.
On AliExpress, choose one that costs $15-$20. Don't pay more for fancier looking ones that cost as much as $100 but are about the same size. Their filtration capacity is the same despite what they advertise. The feature to clean the filter by a rubber lip that turns with the housing is not that helpful. The simpler design of the $15 unit is better overall. I suspect the more complex design is marketing gimmick rather than function.
Cartridge Sediment Filters
micro filtration is 0.1 - 10 µm
Minimum filtration size by cartridge type:
| meltblown PP | 1 micron | gopani.com |
| string-wound | 0.5 micron | gopani.com |
| pleated | 0.1 micron |
The micron rating will either be absolute or nominal.
Absolute – providing assurance that 99.9% of particles at or bigger than the micron rating will be stopped by the filter.
Nominal – these filters are given a percentage rating which indicates the proportion of particles at or bigger than the micron rating that will be trapped by the filter. envirogengroup.com
You will only find pleated big blue filter cartridges of 0.1 micron rating by asking around from sellers on Alibaba or Made-In-China. They can make custom products.
Membrane Filters
ultra filtration is 0.001 - 0.1 µm
A very effective method to remove tannin color is by using ultrafiltration (“UF”) membrane systems. UF systems can be used by homeowners, small communities and commercial sites to reduce turbidity and produce crystal clear water less than 0.1 NTUs. Care must be taken to properly pretreat the water if UF is used if iron, manganese or hardness minerals are present. cleanwaterstore.com
Membrane types:
MF Micro Filtration
UF Ultra Filtration
NF Nano Filtration
RO Reverse Osmosis
Reference:
Membrane Filtration Selection Chart, oregon.gov (pdf)
Membrane Filtration, mrwa.com (pdf)
https://en.wikipedia.org/wiki/Nanotube_membrane
Different Membrane Materials
PVC, PES, PVDF
Sequential Order for Filter Types
Is there a preference for placing a carbon filter before or after a UF membrane? I believe both UF membranes and reverse osmosis membranes leach toxins into the water, thus requiring a “taste polishing” carbon stage after the membranes. If there is a whole house UF membrane, the post carbon stage could be placed at point-of-use.
Reverse osmosis membranes need protection from chlorine, and this may also be true for UF membranes, thus requiring a carbon stage before the membranes.
Of all the cleaners studied for the PE UF membrane, sodium hypochlorite 0.02% available chlorine) was the most effective, and, at the same time, it induced the degradation of the studied membrane the fastest. Structural Changes and Operational Deterioration of the Uf Polyethersulfone (Pes) Membrane Due to Chemical Cleaning, Malczewska and Zak 2019
But what about unchlorinated water? Would you bother adding a carbon filter before the membrane?
While I don't know all the details, the hint that membrane fouling is the bane of existence for RO filtration system owners, tells me that there should be a carbon stage before the membrane.
If the pre and post carbon stages are the same by system design, the post carbon filter can then be rotated to the position before the membrane as it ages.
An aged filter no longer absorbs all toxins, but still has capacity to absorb some toxins. So my current hypothesis is that rotating twice as often may be favorable to replacing both pre and post carbon filters at the same time. Will search for scholar articles on carbon filter stage rotation.
Product Examples
This alibaba $70 filter membrane looks interesting. It's huge at 110cm x 30cm x 30cm, and 15kg (see package and delivery). I could probably set up waste water flowing out of the system like with an RO membrane? Or perhaps just a backwash like in a spin-down filter?
Activated Carbon
A carbon block should not be acting at all as a sediment filter. Activated carbon acts by adsorption, forming bonds with impurities in the water, so trapped sediment will inhibit the carbon from doing its job by blocking water passageways. Thus, the prefilter leading to the carbon block should be at least the same micron rating as the carbon block.
“Charcoal carbon filters are most effective at removing chlorine, volatile organic compounds (VOCs), taste and odor. They are not effective at removing minerals, salts, and dissolved inorganic substances.” 1) Dissolved inorganic substances such as arsenic and asbestos are only partially reduced.
Activated carbon removes a number of specific contaminants from drinking water, including:
Organic chemicals like chlorine tastes and odors
Volatile organic compounds (VOCs)
Lead and maybe other heavy metals
PFOS (perfluorooctanesulfonic acid)
Trihalomethanes (THMs)
Pesticides & herbicides
Contaminants that people most frequently want removed that are not readily removed by carbon filtration are fluoride, nitrates, and sodium. Reverse osmosis and distillation remove all three, so either combined with a high quality carbon filter provides complete treatment. All three can also be removed by selective, non-carbon filters designed for the purpose. For example, you can obtain a double filter with one fluoride and one carbon cartridge if fluoride removal is desired.
…
Timely cartridge replacement is very important, because filter carbon has different capacity for different contaminants. Many people rely on chlorine removal tests to determine when filter carbon should be renewed. This works only if chlorine removal is all you expect from the filter. Most carbon filters will begin to “leak” other chemicals long before they begin to allow chlorine to pass. For example, the MatriKX CTO Plus extruded carbon block used in most PWP RO units and carbon filtration units has an amazing 30,000 gallon chlorine removal capacity (when operated at 1 gallon per minute), but the same filter loses its effectiveness at trihalomethane removal at about 750 gallons. It should, therefore, be replaced annually although it will still have lots of chlorine removal capacity left. ethicalh2o.com
Some chemicals experience “displacement” during carbon adsorption. This happens when chemicals with varying affinities to the carbon media are in play.
Chemicals experience adsorption by making Carbon-to-Carbon (C=C) bonds with the carbon media. The greater number of C’s in its chemical structure, the greater number of C=C bonds the chemical can make with the carbon media, having a greater affinity to it. If a chemical that has a very weak affinity for activated carbon (such as methyl alcohol, with only 1 Carbon in its structure) is paired in an application with a chemical that has a higher affinity (like xylene, with 8 Carbons in its structure), then the xylene, with greater carbon affinity, will be trapped more effectively, and it can actually bump the methyl alcohol off of the carbon media, releasing it into your laboratory atmosphere. labconco.com
Two filters arranged in sequence ensure that any chemical that might get past the first filter is trapped by the second. When the first filter is used up, the second filter is moved to the first position and a new filter is placed in the second position. Water Treatment Using Carbon Filters: GAC Filter Information, Minnesota Department of Health
When will I know my Carbon Filters are Saturated?
Automated Sensor or Manual Detection.
The estimated filter capacity is helpful information, but in order to maintain a safe laboratory environment it is critical to know when it is time to replace the filters. The two primary means of determining filter saturation are by manually monitoring the filters, or by relying on the system’s automatic filter saturation alarm to indicate saturation.
Manual filter monitoring is done with the use of chemical detector tubes and an air sampling device, which allows the user to test the exhaust air of the system using a chemical color indicator tube to determine if chemical breakthrough is occurring. Alternatively, many Carbon Filtration Systems do also have an automated Filter Saturation Alarm on them, which can audibly and visually alert the user when chemical filter breakthrough is occurring. labconco.com
Silver impregnated carbon filters
Activated carbon filters trap organic substances. Trapped organic substances create a breeding ground for microbial flora, which is not good. The solution is to use a Bacteriostatic silver activated carbon to inhibit the growth of microbiological matter and biofilms trapped inside the carbon water filter. thecleanwaterco.com.au
Reverse Osmosis
Reverse Osmosis vs Ultrafiltration, freshwatersystems.com
What is Not Removed by Reverse Osmosis?
Because there are some contaminants that are molecularly smaller than water, Reverse Osmosis isn’t always the silver bullet many people expect when it comes to providing water that’s completely free of impurities. For example, some common contaminants that can slip through the average RO filter are:
- Pesticides
- Herbicides
- Many other agricultural treatment products like fungicides
- Some dissolved gasses, like hydrogen sulfide
- Certain organic compounds
- Chlorine — RO can remove various quantities of chlorine, but there is a possibility that the average home RO filter may not have the capacity to capture all the chlorine present in water, though this will largely depend on the chemical’s concentrations in the water supply.
Reverse osmosis membranes are the primary technology for desalination and wastewater reuse, but they are prone to fouling and subsequent performance deterioration due to poor tolerance to disinfecting agents such as chlorine. Finally, a reverse osmosis membrane not vulnerable to active chlorine, Zhang 2020
When filtering chlorinated water, a carbon stage helps protect the reverse osmosis filter from chlorine.
With unchlorinated water, I still need to protect the RO membrane from damage by: sediment, bacterial growth, rust, oil, and drying out 2). “4 main causes of fouling – suspended solids, organic, scaling, and biological – that effect RO and NF membrane performance” 3) “The bane of existence for users of reverse osmosis (RO) membrane systems (Figure 1) is controlling membrane fouling from microorganisms.” 4)
Micro-filtration (0.1 to 5 microns) can be used for separation of oil/water emulsions 5). 0.1 micron filtration will also remove bacteria. Thus 0.1 micron filtration will protect the RO membrane from all dangers mentioned for unchlorinated water. A carbon pre-filter doesn't appear to be necessary for my use case.
To further simplify the RO system, I can choose tankless. This also cuts out the need for a post RO carbon stage, because this carbon stage is for removing the taste imparted by the holding tank.
Even without a pressurized tank, a check valve is needed? “A check valve is often located directly after the RO membrane to prevent any backflow through it. It can be seen in Figure 3 as valve #5. If there is a prevention of flow downstream by either a closed valve or pipe, then osmotic pressure will cause this purified water to travel back through the RO membrane, which could cause damage to the system.”. 6)
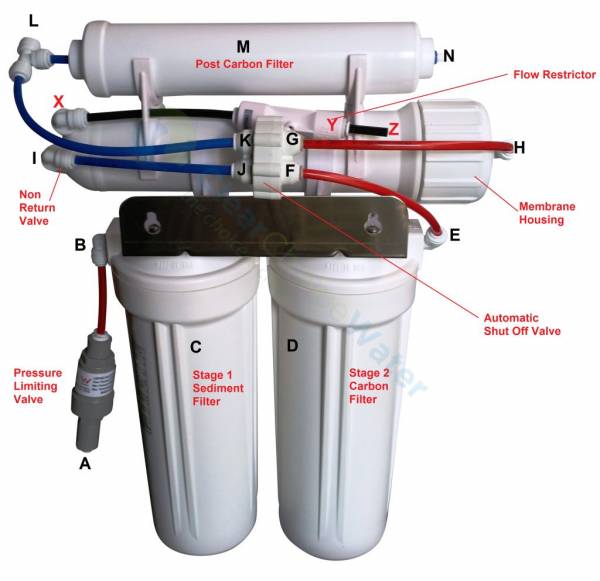
To get a decent flow rate in filling a glass without a pressurized tank, I can choose a 3213-800 membrane, capable of 800 gallons per day, which amounts to 2 liters per minute.
On AliExpress, any 800gpd system is insanely expensive, so I have to order each part separately and assemble:
Membrane $50
Housing $50
Flow Restrictor $5
ASO valve (automatic shutoff)
Flow Restrictor size calculator tells me 6307 ml/min if the PDR is 3 (production to drain ratio). The highest flow, flow restrictor I could find was 1500cc, so I will be needing 4 of those in parallel.
Solar Still
Solar powered distillation:

Sustainable Energy Technologies for Seawater Desalination, Rosen and Farsi 2022
Contaminant Testing Kits
“What Should I Test For? A number of tests can be performed to check for specific water contaminants. To test for all possible pollutants, however, would be prohibitively expensive. Instead, tests should be conducted for the most common problems or for suspected problems.” okstate.edu
Oklahoma State University gives tables to help you figure out what tests to conduct.
Henry Boyter on Quora hoped government or UN assistance would provide a test like the following:
However, this is a one time test? Without assistance the cost is prohibitive at $300 in 2018 and $390 now. I want to test frequently and also test after filtration.
I was fortunate that the town just recently paid to have their water tested. A separate page mentioned samples were taken at the mains and 3 house taps.
Local Clinic Water Quality Test
Implementation
Now it's a matter of finding the combination of filters that will do the minimum required job at the least expense. Options include sediment filters, ion exchange, activated carbon, ozonation, reverse osmosis, etc.
I think most affordable for a large consumption of drinking water would be reverse osmosis. For just one person, then a well designed pitcher filter is more economical. My hypothesis is that filter cartridges and membranes have a limited lifespan while sitting in the system, and should be replaced after some time regardless of the amount of water filtered. Related question on Quora.
Avoid proprietary and use standard filter sizes
First implementation with whole house 1 micron filtering
Drinking water filtration to combine with 1 micron whole house filtering
The first implementation plan was:
- 1 micron whole house filtering
- zero-water pitcher
- sprite shower filter
With 1 micron filtering, the house tap water looks murky and the toilets look soiled quickly from the sediment/tds.
Possible filter damage due to air hammering
1 micron isn't enough.
UF UltraFiltration membranes don't work inside sediment filter housings
On Alibaba I talked to Vickie Yang with Guangzhou Lvyuan Water Purification Equipment. She recommended pleated 0.1 micron filters with a 5 micron carbon core for $23 each.
Images of pleated filter with carbon core
Image of resulting water clarity from house tap
Reduced Overall Cost in just having a single filter
Avoid Customs Clearance Fees from Courier
To remove plastic related toxins, I will still implement point of use filtering. Water going past the whole house filters sits in a plastic storage tank and flows through the solar hdpe irrigation hose and pvc plumbing. The whole house filtering will help extend the life of the point of use filters.
The second implementation plan is:
- 0.1 micron pleated with carbon core, whole house filtering
- zero-water pitcher
- sprite shower filter
The sprite shower filter has a proprietary Chlorgon filter which relies on copper, zinc, and calcium sulfide to remove contaminants. spritewater.com.
AliExpress alternatives to Sprite brand
For drinking water, I want to know more about filtration of PFAS and plastic related toxins. Or whatever I could expect. If the zero-water pitcher is not ideal, I could choose a carbon and RO system.
Would also be useful to go over water test results to see what toxins are in the water, and what filters would work best for those toxins.
Plumbing and Maintenance
To save on cost by extending filter life, the filter stages can be backwashed. Backwashing is possible, as mentioned in this manufacturer video. Although I don't know how to prevent microorganisms from growing on filter surfaces over time? See: https://www.quora.com/In-addition-to-backwashing-can-you-periodically-sanitize-sediment-water-filters-of-microorganism-growth-If-so-what-methods-do-you-use
In addition to backwashing, you can refresh a carbon block a few times by baking at 300F. hunker.com. Or by boiling. outie555 on YouTube.
There are some industrial spent-carbon indicators. These would be helpful in knowing when it's time to change or refresh the carbon stage. However, there aren't many options for consumers. The sprite shower filter includes an inspection window for the media to detect color change.
In order to be able to backwash filters individually, a network of pipes and valves are needed as follows. Normal house usage flow is left to right. The circles are symbolic representations of the filter cartridges, and the rectangles are shut-off valves. The last valve to the right is recommended so no one can use water in the house, interrupting your work at flushing filter cartridges.
Due to house configuration, water flow in the image below is right to left. Note there are 2 white quick disconnect fittings (though I can't remember what for).
Before backwashing, purge water out from the incoming pipe until the water runs clear. In normal use the water is slow running and sediment will accumulate in the pipe leading to the filters.
When replacing a filter, or when servicing, reapply food grade silicone grease or petroleum jelly. Also pour a little bit of water with bleach at the bottom. GG&T video.
I've bought some filter cartridges that are about 5mm too short, even with the compression gaskets (rubber washers) on each end. Once you install the cartridge and tighten the housing, you can know the filter isn't seated/sealed because it rattles when you shake the housing. I used the rubber washers from another filter and stacked them 2 high on top of the filter cartridge. Silicone grease or petroleum jelly helps stick the washers together so they stay in place during installation. With a total of 3 washers (1 below and 2 above), there was a slight inprint on the top washer upon removal.
I then tried 4 washers (2 below and 2 above) and though I feared I wouldn't be able to tighten the o-ring seal, there was no leak at pressure. Still not sure if 3 or 4 washer stack height is best, depending on how much the top housing protrusion should bite into the rubber washer (aka compression gasket).
Water Chlorination
The Future of Chlorine, Robin Hackett, utilityweek.co.uk 2018
How Do You Like Your Tap Water, Ortiz et al 2016